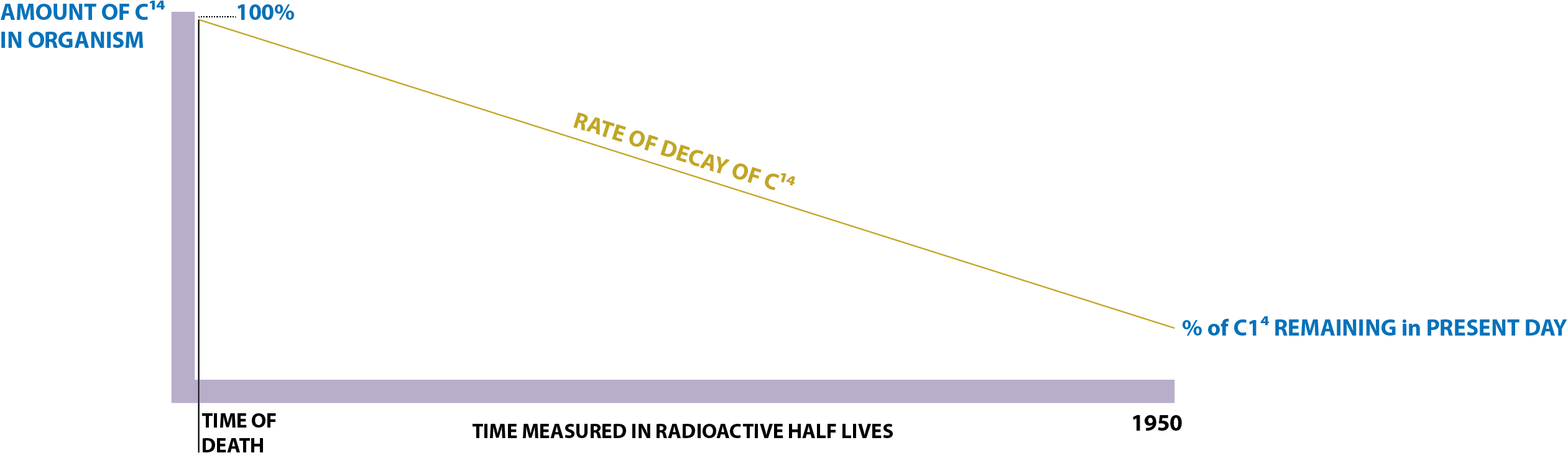
Scientists can measure how old remains of plants or animals are by measuring the ratio of carbon-12 and carbon-14 in their remains (radiocarbon dating). Organisms take up carbon while living but stop when they die. Carbon-12 is a stable isotope, and carbon-14 is an unstable radioactive isotope that decays over time. The half-life of carbon-14 is 5,730 years, meaning that half of the carbon-14 what was in a sample has decayed into the stable form of nitrogen-14 when the sample is 5,730 years old. By measuring how much carbon-12 and carbon-14 are in a sample, the date of death can be known. For example, this date would be the year the tree was cut down to make the canoe in the exhibit.
What is radiocarbon?
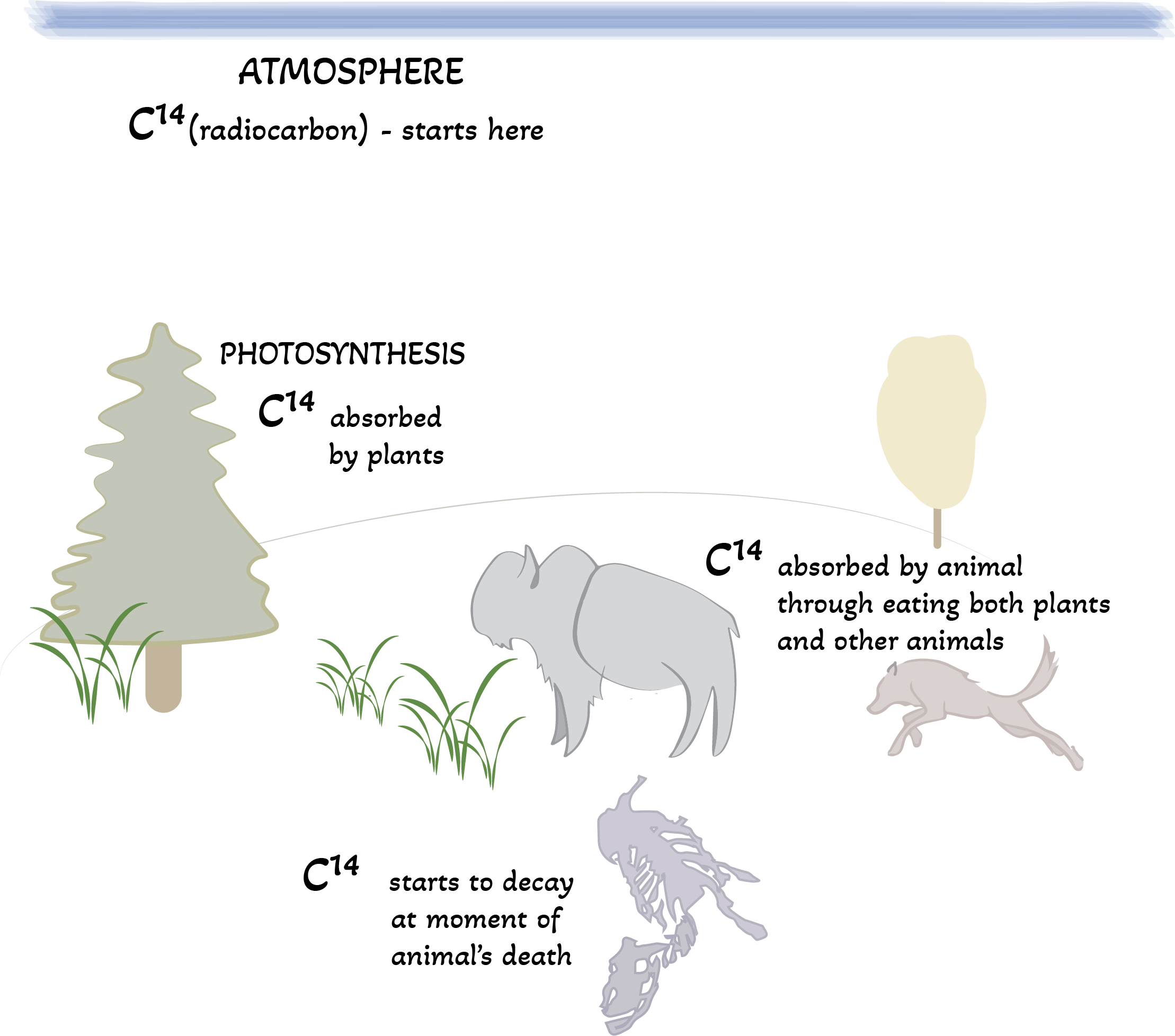
Radiocarbon is a radioactive isotope of carbon (C14). Most of the radiocarbon found on earth is formed naturally in the upper atmosphere from high-energy cosmic rays (from outside the solar system) that are constantly bombarding the upper atmosphere.
- Plants use photosynthesis to absorb the radiocarbon (C14) in our atmosphere.
- Animals (herbivores and omnivores) absorb C14 by eating plants. All living organisms have the same radiocarbon as the atmosphere, just like plants.
- All animals in the food chain get C14 directly (eating plants) or indirectly (eating animals which eat plants) from plant material.
Once an organism dies the radiocarbon is no longer replaced so the C14 starts to slowly decay and eventually becomes Nitrogen (N14). The rate of decay is constant which is why radiocarbon dating is so effective and reliable.
The material used for radiocarbon dating must once have been part of a living organism.
Scientists cannot use:
- Stone
- Metal
- Pottery (unless there is some organic material embedded or left as a residue)
A radiocarbon date tells when an organism was alive (not when the material was used). This fact should always be remembered when using radiocarbon dates.
The date range that radiocarbon dating can detect is a few hundred years ago to ~ 50,000 years ago
Note: In general it is always better to date a properly identified single entity (such as a cereal grain or an identified bone) rather than a mixture of unidentified organic remains.
Common materials for radiocarbon dating are bone, animal, wood, tree, charcoal, linen, flax plant, wool, and parchment.
Radiocarbon measurements are always reported in terms of years `before present' (BP). "BP" is calculated on the assumption that the atmospheric radiocarbon concentration has always been the same as it was in 1950 and that the half-life of radiocarbon is 5568 years. For this purpose, the `present' refers to 1950.
Some things will mess up the accuracy of a radiocarbon date.
Contamination:
Material from the soil or conservation work can become incorporated into the sample resulting in an admixture of carbon with a different radiocarbon content and skewing the accuracy of the date.
Artificial effects: Radiocarbon is also formed (through the processes described above) in manmade nuclear reactions. There is some produced in nuclear power generation, but this is a relatively small effect. The amount produced in the atmospheric nuclear bomb tests of the 1950's and 1960's was, however, large. For a short period, the abundance in the atmosphere was almost doubled. This means that it is very easy to distinguish pre and post bomb organic material by making a radiocarbon measurement.
The other major artificial effect relevant to radiocarbon dating is the effect of fossil fuel burning. This releases a large amount of carbon dioxide into the atmosphere that contains virtually no radiocarbon (because the organic material from which it derives is so ancient). This reduces the ratio of radiocarbon to stable carbon in the atmosphere. Partly because of this, it is very difficult to radiocarbon date material from the period 1650-1950.
Radiocarbon dating was developed in the late 1940s at the University of Chicago by Willard Libby.
University of Chicago - article "Carbon-14 dating, explained"
----------------------------
Additional info: Australia National University School of Earth Sciences - Radiocarbon Lab website